Chiara Bernard Ph.D. Candidate 36th cycle, University of Trento, DICAM
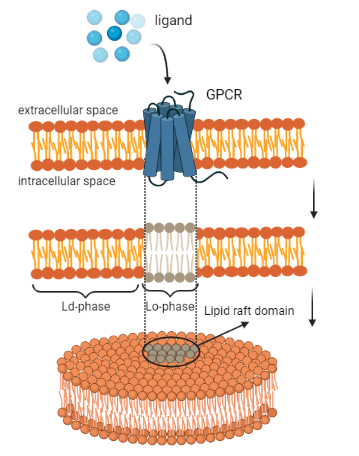
The study of the lipid bilayer membrane plays a pivotal role in the understanding of many biological mechanisms connected to cellular homeostasis and metabolism. In particular, the lipid membrane is a semipermeable structure that regulates the transport of nutrients inside and outside the cell. It is composed by amphiphilic phospholipids with a polar head group and two hydrophobic tails. The latter ones are responsible for the bilayer phase transition from a liquid-disordered to a liquid-ordered phase, also called the "raft phase". These two stages depend on lipid tails organization that implies different membrane composition and thickness.
The thicker phase is characterized by microdomains composed by sphingolipids and cholesterol that segregate transmembrane proteins. These microdomains are called lipid rafts and they are predicted to nucleate, grow, and evolve through receptor’s activity and their interactions with pairing proteins. The importance of studying the mechanobiology behind lipid rafts formation can support the understanding of different membrane-mediated processes through which cells communicate with the extracellular environment. Among these, for instance it has been demonstrated that lipid rafts represent an entry port for viruses such as Poliovirus and SARS-CoV [1].
Recent findings demonstrate the role of lipid-rafts as ligand’s entry-mediators within cells [2]. Hence, a broad comprehension of membrane mechanisms can lead to remarkable implications in the study of viral lifecycle, virus-cell interactions, and viruses’ uptake. Mechanics of lipid rafts is studied for laboratory-made monolayers and bilayers [3]. In fact, mechanobiology allows for explaining why certain ligand-receptor activity triggers lipid raft formations across the cell membrane. Common and established knowledge is that ligand-receptors complexes form only by allowing conformational changes of the active receptors.
The mentioned investigation [2] is performed by describing the conformational change of lipids through a continuum-based approach involving the remodelling of G-Protein Coupled Receptors (GPCRs). This arises through the interplay of mechanical balance (involving elasticity, chemical potentials of the interacting species and their remodelling), tendency of the membrane to evolve towards minimal energy configurations and reaction-diffusion dynamics of receptors and transporters species modelled by means of interspecific interactions. In particular, the activity of GPCRs is estimated by measuring cAMPs (cyclic Adenosine Monophosphate) concentration inside and outside the cell so obtaining an experimental way of calibrating the parameters in the evolutionary equations of the coupled model.
Through in silico simulations, our purpose is to investigate this process in depth to have a quantitative predicting model for characterizing the space-time changes of rafts domains within the cell membrane to effectively observe the co-localization and synergy between the activation of membrane proteins and rafts formation, also including the influence of other membrane molecules such as cholesterol.
This could contribute to the attempts of testing/designing new or existing drugs by selectively controlling the mechanical properties and selectivity of the membrane to external agents, so orienting intracellular functions.